REVIVE: Reducing Exsanguination Via In-Vivo Expandable Foam
The purpose of this page is to inform the public about a clinical trial in which patients with internal bleeding due to trauma may be enrolled without prior consent.
Informed consent is the process where the researchers explain a research study with a person to help them decide whether or not to take part in the research study. Taking part in research is voluntary and people have the right to refuse to take part in a research study. In some emergency situations, people who are taking part in a research study are not able to give their consent due to their condition. The REVIVE trial is a research study in which patients with severe internal bleeding may be included in the study without their consent.
What is the purpose of this research study?
Trauma, which is a severe injury usually caused by violence or road traffic accidents is the leading causes of death in people younger than 45 years. Trauma patients may have extensive internal bleeding. People who suffer severe trauma usually need emergency care to stop bleeding. Internal bleeding in the abdomen (belly area) is a difficult problem. In most patients who are severely injured, the only way to stop the internal bleeding in the abdomen is by major emergency surgery. Almost half of the patients who suffer from abdominal bleeding die even after they reach the hospital because they had too much bleeding. There is an urgent need for new ways to treat patients with bleeding in the abdomen.
A company called Arsenal
This research study is funded by Arsenal Medical and
Why is it not possible to get
People who have had severe internal bleeding are usually in shock due to
The study doctors and study staff will make attempts to reach family before enrollment. If patients are enrolled into the study before consent was obtained, the study doctors and study staff will talk with the patient and/or family as soon as possible after the procedure to obtain consent for continued participation in the research.
Who will be included in this research study?
Trauma victims who have had severe internal bleeding in the abdomen who need immediate surgery and may not survive transportation to the operating room will be included in this research study. We plan to include people who are at least 16 years old.
What are the study procedures?
When a trauma patient arrives in the emergency department the emergency doctors will examine the patient and decide if they meet the criteria for being in this research study. If the doctors confirm that the patient is in shock due to severe bleeding in the abdomen and is likely to die without emergency surgery, the doctors will deliver ResQForm into the abdomen through a nozzle. The patient will then be immediately transported to the operating room. In the operating room, the foam will be removed and the patient will be given usual care for treatment of the injuries. Information about the injury and care given until discharge from the hospital will be collected for the research study.
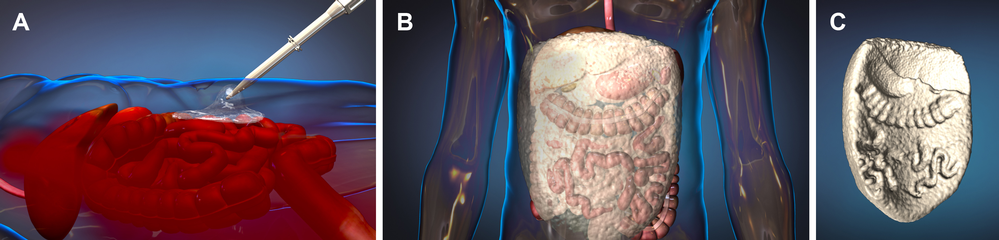
What are the risks of using ResQFoam?
The possible risks of using ResQFoam are
How many patients will be included in this research study?
We plan to include up to 40 trauma patients at 4 to 5 hospitals around the country. At UTHealth, we plan to include about 15 people in this research study.
When will the study start?
We plan to start this research study in the spring of 2018 and the study is expected to run for about 2 years. We hope to complete the study by early 2020.
If I do not wish to be included in this research study, how can make my wish known?
If you do not wish to participate in this research study, please email us at [email protected]. We will be happy to provide you with an ‘opt-out’ bracelet that you should wear for the duration of the study.
For more information about this research study visit NCT02880163.