Description
A Coverage Analysis is a systematic review of all procedures detailed in the study protocol to determine how each service and/or procedure at each visit time point should be billed to ensure institutional billing compliance. Medicare billing guidelines are referred to during this determination process.
UTHealth Houston has created the “Coverage Analysis/Internal Budget” (CAIB) tool to record the billing coverage analysis determinations for all clinical studies conducted at the institution.
- Coverage Analysis tab lists out all services and procedures that are detailed in the study protocol and notes
Will this service or procedure generate a bill in billing system?
If yes:
-
- Should it be billed to the patient/patient insurance?
- Should it be billed to the study account?
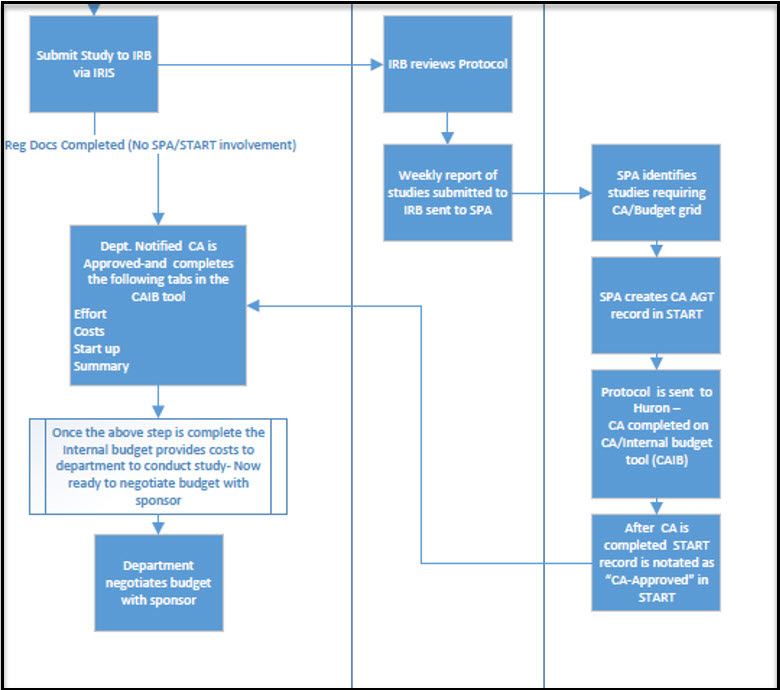
Cost Analysis Flowchart
Coverage Analysis Summary Process Outline (Text Alternative)
When the analysis is complete, the investigator/study team has a study-specific billing summary to guide the study team with clinical billing for research visits.
Note: This text provides an accessible outline and table of contents for the process shown in the accompanying flowchart image. It describes the steps involved in submitting, reviewing, and approving a clinical study budget.
1. Submit Study
Submit study to the IRB (Institutional Review Board) via IRIS.
2. IRB Review
- The IRB reviews the protocol.
- A weekly report of studies submitted to the IRB is sent to SPA (Sponsored Projects Administration).
3. SPA (Sponsored Projects Administration) Actions
- SPA identifies studies that require a CA or Budget Grid.
- SPA creates a CA AGT record in START.
- The protocol is sent to Huron, where the CA is completed in the CA/Internal Budget Tool (CAIB).
- After completion, the START record is updated to show
CA-Approved
status.
4. Department Notification and CAIB Tool
Once notified that the CA is approved, the department completes the following tabs in the CAIB tool:
- Effort
- Costs
- Start-Up
- Summary
5. Internal Budget Development
After completing the CAIB tool, the internal budget provides cost details for the department to conduct the study. The department is now ready to negotiate the budget with the sponsor.
6. Budget Negotiation
The department negotiates the study budget with the sponsor based on internal cost estimates.
Summary of Roles and Flow
- Department: Completes CAIB, prepares internal budget, negotiates with sponsor.
- SPA / IRB: Reviews protocol, identifies CA requirements, and updates START and Huron systems.
- Process Flow: IRB submission → SPA review → Department budget preparation → Sponsor negotiation.
This text serves as a fully accessible equivalent of the flowchart image for users of assistive technology.
Process
To conduct a coverage analysis, a thorough review of all study documents, clinic procedures, and national billing guidelines must be performed. At UTHealth Houston, this starts with the billing risk review by the Clinical Research Finance and Administration (CRFA) team. Each week, the CRFA team reviews all protocols submitted to the Institutional Review Board from the prior week. While reviewing the protocol, CRFA analyzes the schedule of activities to determine if any activity could generate a charge in the clinical billing system. If a study has an activity that could generate a bill, it is designated as a billing risk.
After identifying a billing risk, the CRFA team then is responsible for ensuring a billing coverage analysis (CA) is built for the study. Effective 01/01/2022, UTHealth Houston utilizes a third party who specializes in billing coverage analyses to build out CAs. This ensures unbiased billing assessments and condition/indication specific billing expertise. UTHealth Houston has contracted with Huron Research Group to build out the coverage analysis which includes notating billing determinations for all services and procedures occurring at each study visit as described within the protocol and providing justification for all items notated as routine/standard care by referencing a national guideline or peer reviewed publication which indicates that the activity is adequate and necessary in the treatment of the patient's condition.
Once CRFA team has received completed CA back from Huron, the CA is uploaded into UTHealth Houston START CA AGT record and the status is changed to 'Approved'. UTHealth Houston START will send an automated notification to the Department that the CA is complete and ready for review. If the Department has any questions or comments, they should notify the CRFA team via email at [email protected] . CRFA team will work with the department and Huron to make changes (if applicable). If department would like to contest final determinations, the CA can be escalated to the UTHealth Houston Billing Audit group.
CRFA team will then send the final Coverage Analysis to the Committee for Protection of Human Subjects for upload into iRIS, and create the Study Administrative Record in Epic (if applicable)
The Coverage analysis (CA) record is created in UTHealth Houston START by the CRFA team. You will be notified via automated email from UTHealth Houston START once the CA for your project has been completed.
-
Fees for Industry Sponsored Clinical Trials
Industry Sponsored studies and departmentally funded studies will be billed for CA builds. The Department must ensure that coverage analysis fees plus IDC are included in startup fees when negotiating budgets with Industry/For profit sponsors. Departmentally funded studies will be billed the Huron fee without IDC. (See: "Coverage Analysis Fees - Industry Sponsored Studies" under located Tools & Resources/Forms - CRF section).
-
Standard of Care -VS- Research Cost
In the terms of the UTHealth Houston Coverage Analysis, standard of care activities are those which the patient would receive regardless of their participation in the study, while research costs are those that being performed outside of this. Another way to think about the difference, is whether or not your normal clinic practice is changing to accommodate the service or procedure. In addition, any activities which are promised as free of charge in the Informed Consent Form automatically become research costs.
Helpful websites for standard of care vs research:
-
Department Tasks / Responsibilities
- Submit study to IRB (This initiates the CA process)
- Review final CA uploaded into START by SPA's CRFA team
- Contact CRFA team via email to [email protected] if any discrepancies are noted.
- Complete internal budget tabs
- See: guidance document "CAIB Tool Guidance Document" located Tools & Resources/Forms - CRF section
- Negotiate budget with sponsor AFTER the internal budget tabs have been completed
- Do not start negotiations with sponsor until you have a clear idea of the costs to conduct the study
- Upload the final completed CAIB tool under the Proposal Record
- See UTHealth Houston START guidance document “Clinical Trials- PD”
-
SPA Tasks / Responsibilities
- Review IRB application for potential billing risk
- Send study documents to Huron Research Group to build out the Coverage Analysis
- Upload completed Coverage Analysis into UTHealth Houston START
- UTHealth Houston START will send automatic notification to the department.
- Send a copy of final Coverage Analysis to CPHS to upload into IRIS
- Maintain all records